Abstract
Objectives: Acute myeloid leukemia (AML) with FMS-like tyrosine kinase-3 (FLT3) internal tandem duplication (FLT3-ITD) is associated with poor response to chemotherapy and prognosis. In vitro drug screening and laboratory mechanistic studies have identified omacetaxine mepesuccinate (OME, also known as homoharringtonine) as an effective agent that demonstrated synergism with FLT3 inhibitors in this AML subtype. We report herein the clinical outcome of the first 40 FLT3-ITD AML patients treated with sorafenib and OME combination (SOME).
Methods: Patients with relapsed or refractory (R/R) FLT3-ITD AML were recruited and treated with sorafenib 200-400 mg b.d. continuously and OME [2 mg daily for 7 (first course) or 5 days (second course onwards) every 21 days] until disease progression or allogeneic hematopoietic stem cell transplantation (HSCT). Complete remission (CR) or CR with incomplete hematologic recovery (CRi), leukemia-free survival (LFS) and overall survival (OS) were evaluated. Partial remission (PR) was defined as marrow or circulating blasts ≤ 50% of the pre-treatment state. LFS and OS were evaluated by Kaplan-Meier analysis and compared by log-rank test. Fisher's exact test was used to compare nominal variables. Cox proportional hazard model was used in the univariate and multivariate analyses.
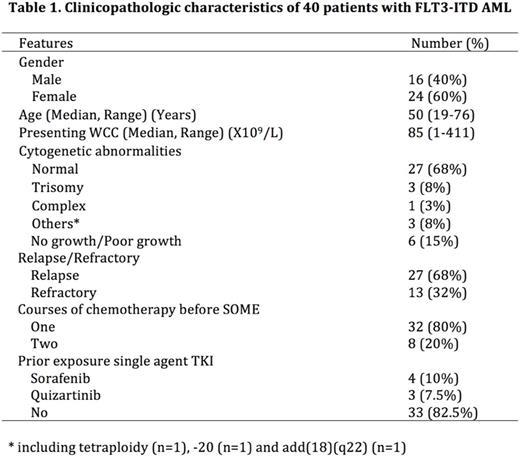
Results: Forty patients (16 men, 24 women) were recruited between June 2013 to July 2017. Their clinicopathologic characteristics were shown in Table 1. Thirty patients (75%) achieved CR/CRi, 2 patients showed PR and 7 patients (of whom 5 have had exposure to FLT3 inhibitors) showed no response. One patient was on day 5 of treatment at the time of writing this abstract. The median LFS of the 30 patients who achieved CR/CRi was 4.0 months. Prior exposure to FLT3 inhibitors ( p =0.003) and more than one induction chemotherapy before SOME ( p <0.001) were associated with a lower rate of CR/CRi. Non-hematological toxicities were minimal and limited to hand-foot-skin reaction and rash associated with sorafenib. Univariate analysis showed that early treatment with SOME (defined as ≤10 months from diagnosis) and HSCT after SOME induced remission were associated with better LFS with a hazard ratio (HR) of 4.1 (1.2-14.1, p =0.026) and 6.1 (1.7-22.0, p =0.005). Achievement of CR/CRi after SOME (HR 4.1, 1.5-11.5 p =0.007), <2 prior induction chemotherapy regimens (HR 3.6, 1.3-9.8, p =0.013), early treatment with SOME (HR 2.8, 1.1-7.2, p =0.031) and HSCT (HR 6.5, 1.5-29.0, p =0.014) were associated with superior OS. In multivariate analysis, HSCT was the only parameter associated with a better LFS (HR 5.4, 1.5-20.0, p =0.012) and OS (HR 6.0, 1.3-27.4, p =0.022).
Conclusion: Combination of sorafenib with OME is an effective and safe regimen for R/R FLT3-ITD AML. The molecular features predictive of treatment response as well as the emergence of tyrosine kinase domain mutations at relapse are being evaluated. Further studies testing combination of OME with other FLT3 inhibitors will soon begin.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal